Bonds are used for explaining cells, and as such a quick explanation is needed:
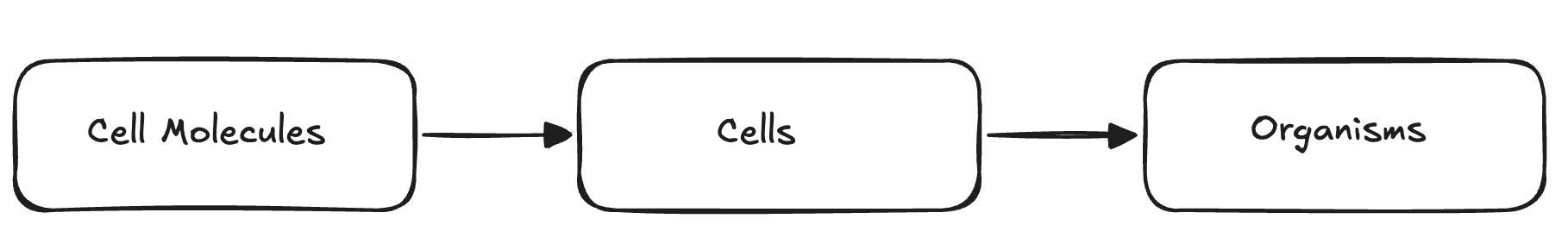
There is a 3 level hierarchy of cells, namely molecules, cells, and organisms. Molecules are the smallest level of organization, and they make up cells. Cells are the basic unit of life, and they make up organisms. Organisms are made up of one or more cells.
Structure Of an Atom
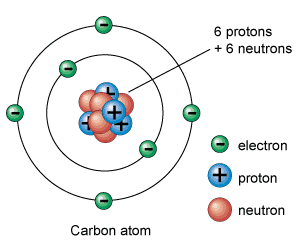
Taking a detour into Physics, we note the nature of Protons, Neutrons, and Electrons. Protons and Neutrons are made up of Quarks. Quarks are the fundamental particles that make up matter.
What we care about is the distribution of electrons around the nucleus of an atom. The number of protons in the nucleus determines the element, and the number of electrons determines the chemical properties of the element.
Despite the incredible diversity of types of atoms, biology only really ever makes use of a few types of atoms. The most common atoms in biology are Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, and Sulfur. These atoms are the building blocks of life, and they make up the majority of the mass of living organisms.
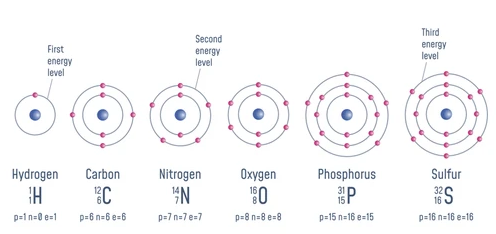
Electron Shells are regions around the nucleus where electrons reside. Examples: Hydrogen 1 (1st shell); Carbon 6, Nitrogen 7, Oxygen 8 (1st-2nd); Phosphorus 15, Sulfur 16 (1st-3rd).
Bonding
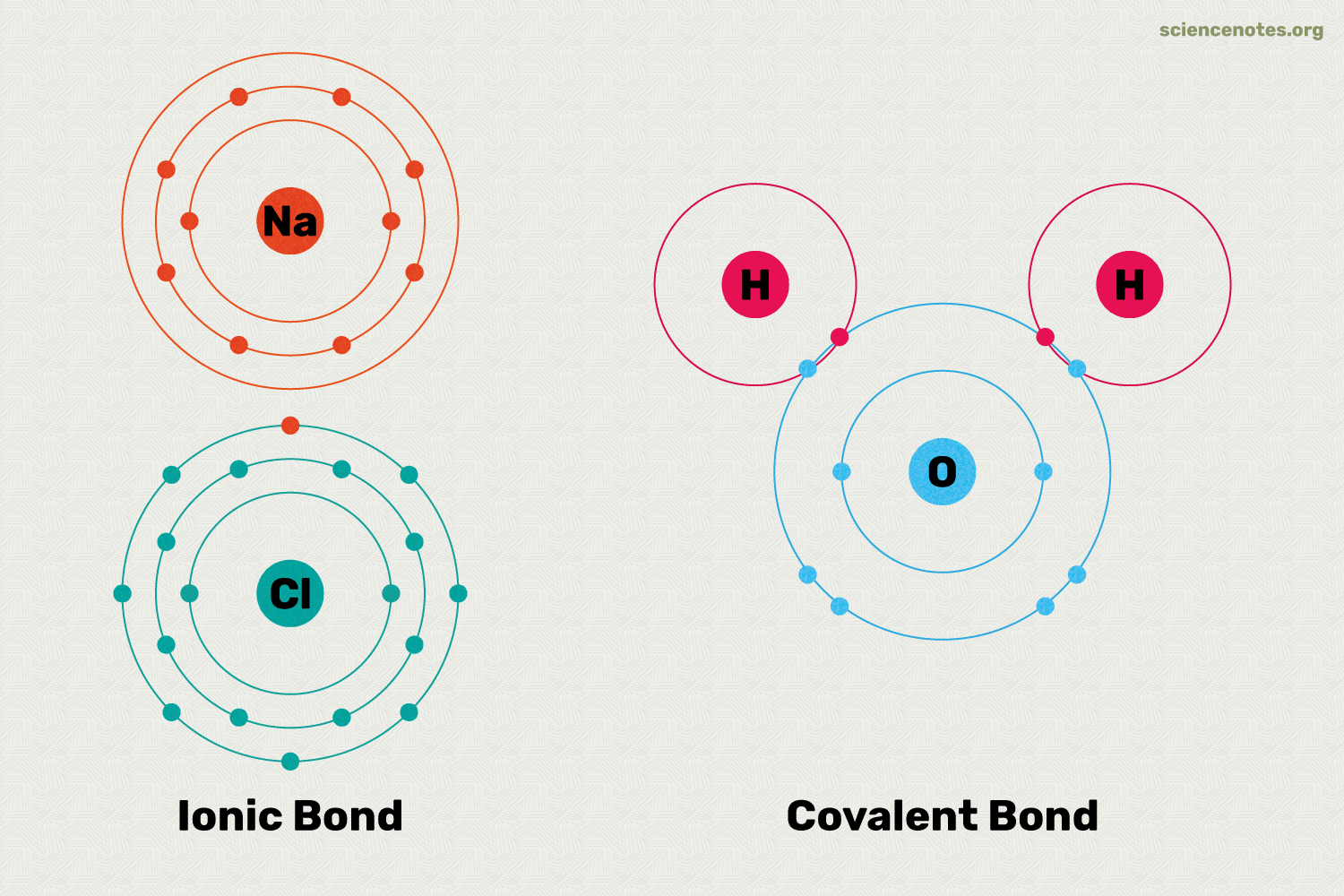
Two atoms can form a bond by sharing or transferring electrons. There are two main types of bonds: Covalent Bonds and Ionic Bonds.
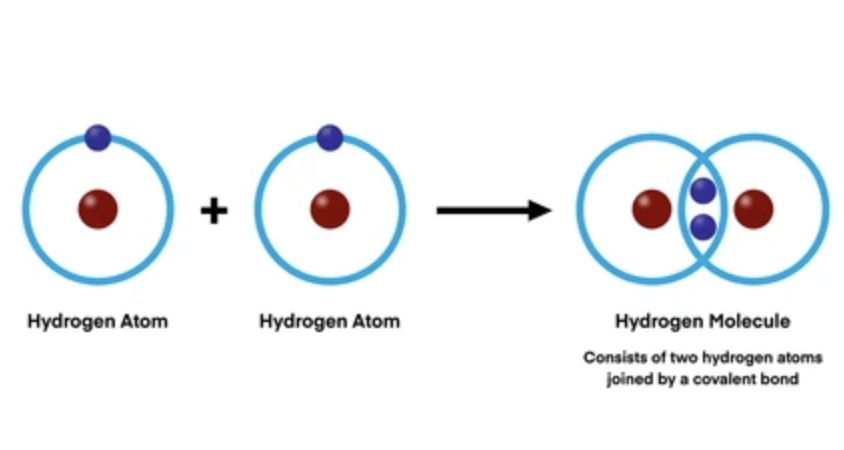
Covalent and Ionic Bonds
Covalent Bonds are formed when two atoms share electrons. This type of bond is very strong and is the most common type of bond in biology. Covalent bonds can be either polar or non-polar. Polar Covalent Bonds occur when the electrons are not shared equally between the two atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other atom. Non-Polar Covalent Bonds occur when the electrons are shared equally between the two atoms.
Ionic Bonds are formed when one atom transfers an electron to another atom, resulting in the formation of ions. The atom that loses an electron becomes a positively charged ion (cation), while the atom that gains an electron becomes a negatively charged ion (anion). Ionic bonds are weaker than covalent bonds and are often found in salts.
Example
Based on what we just discussed, covalent bonds between atoms wtih similar electronegativities are non-polar bonds, and the covalent bonds between atoms with different electronegativities are polar bonds.
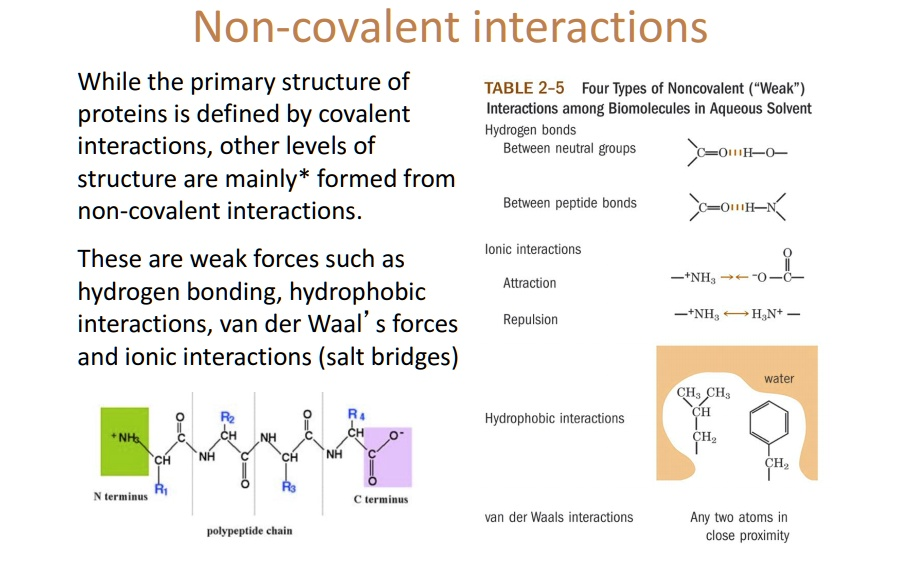
Non-Covalent Bonds
Non-Covalent Bonds are weaker than covalent and ionic bonds, but they are still important in biology. They are distinct because they can connect different molecules together, whereas covalent and ionic bonds connect atoms within a molecule.
FYI
In the context of chemistry, ionic bonds are said to be stronger vs. covalent bonds. This is true in a theorectical sene, but only between individual ions and not when there’s water involved (which there always is in cells!)
Types of Molecular Bonds
These distinct types of bonds are important because they determine the structure and function of molecules in biology.
Hydrogen Bonds
Hydrogen bonds are a type of non-covalent bond that occurs when a hydrogen atom is covalently bonded to a highly electronegative atom (such as oxygen or nitrogen) and is attracted to another electronegative atom. Hydrogen bonds are important in biology because they help to stabilize the structure of proteins and nucleic acids.
Example
hydrogen bonds between water molecules
Van Der Waals Interactions
These interactions are charecterized by a VERY TEMPORARY opposite charge on two atoms that are very close to each other. These interactions are important in biology because they help to stabilize the structure of proteins and nucleic acids.
Example
Van der Waals interactions between the non-polar regions of molecules
Question
Think about the three main types of noncovalent bonds - hydrogen bonds, ionic bonds, and van der Waals interactions. Which type is the strongest? Weakest?
Answer
The strongest type of noncovalent bond is the ionic bond, followed by the hydrogen bond, and the weakest type is the van der Waals interaction. However, it’s important to note that in biological systems, the strength of these bonds can be influenced by the surrounding environment, such as the presence of water.