Water, in the context of biology, is a fundamental molecule that plays a crucial role in the structure and function of living organisms.
Atomic Structure
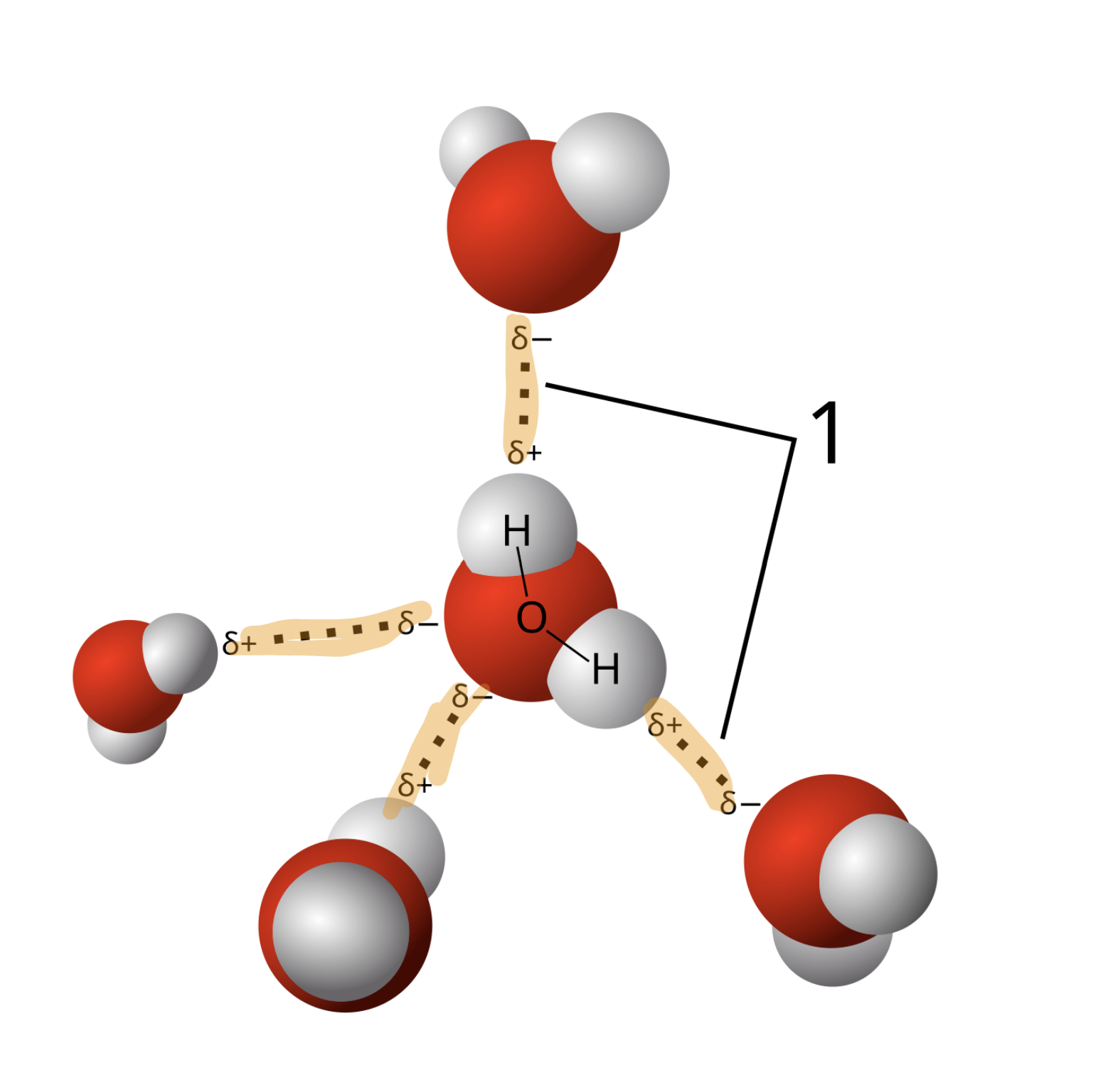
A water molecule has equal numbers of partial negative and partial positive charges (two of each).
Partiall charges spread out as far as possible in 3D space.
Each water molecule can form hydrogen bonds with up to 4 other water molecules.
Unique Properties
The fact that so many hydrogen bonds can form between water molecules explans many of water’s unique physical properties.
For one, it has a very high heat capacity, meaning it can absorb a lot of heat without a significant increase in temperature.
Example
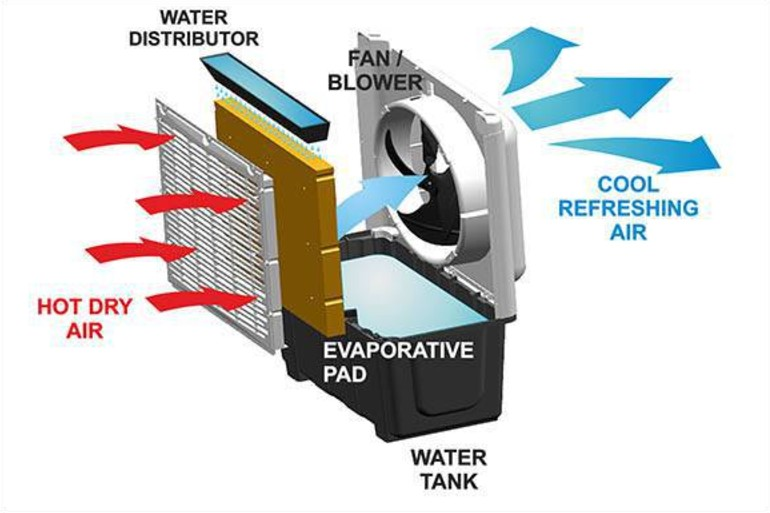
evaporative cooling - lots of hydrogen bonds between water molecules means it takes a lot of energy to pull them apart
Example
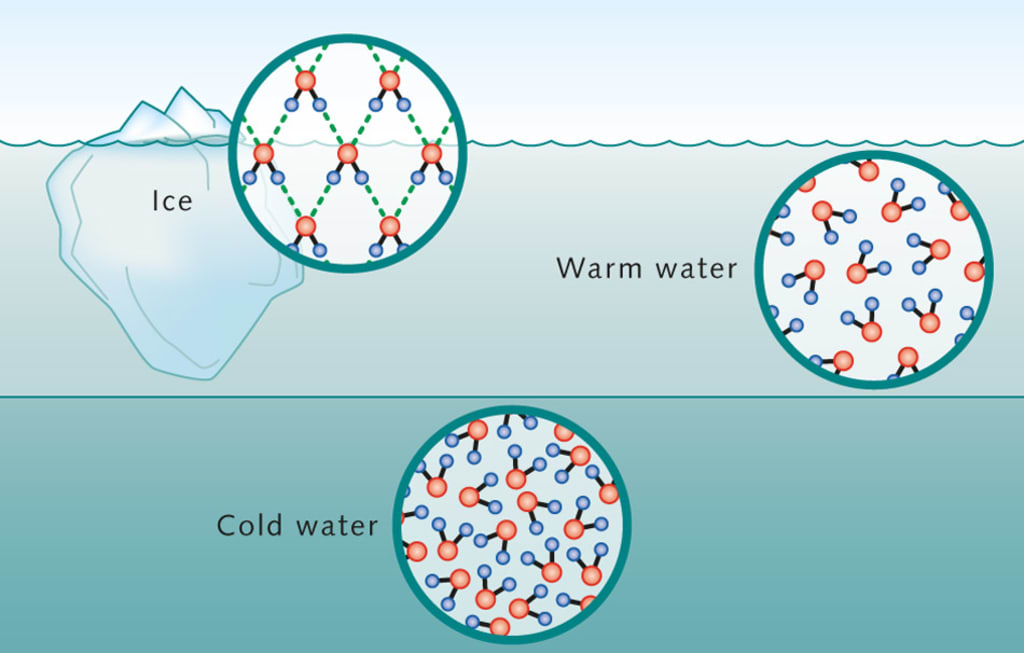
expanding when freezing - hydrogen bonds hold water molecules apart in a crystalline structure